
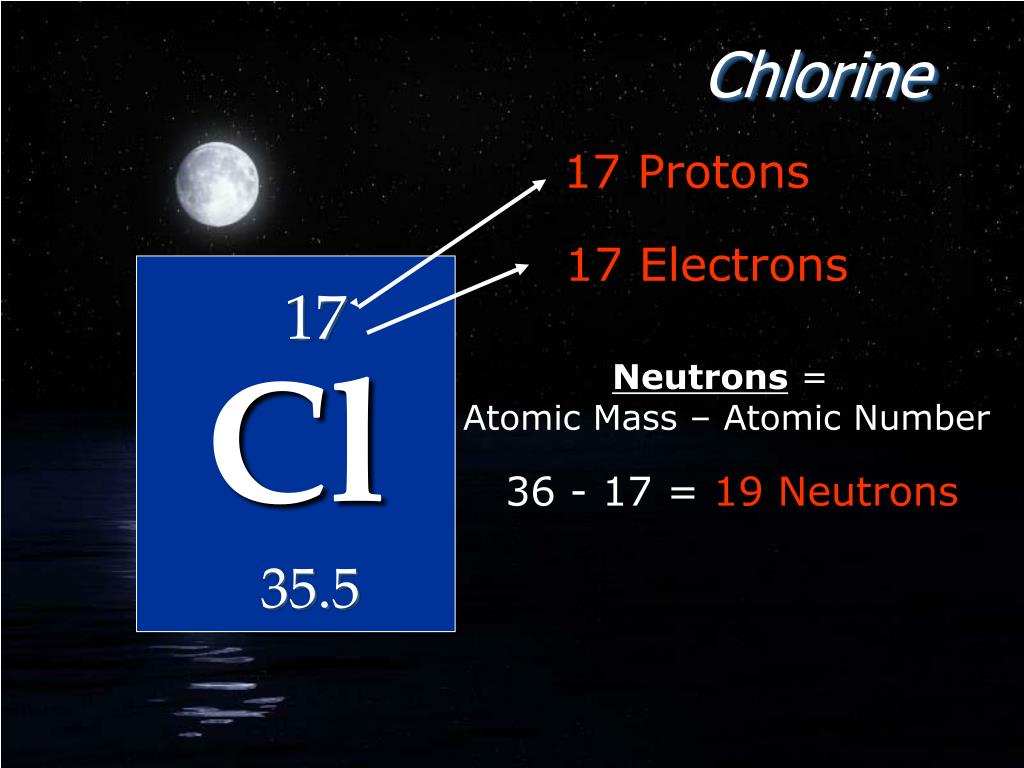
The average mass of an elements naturally occurring isotopes compared to the mass of an atom of 12 C is defined as its relative atomic mass. For example, in Cl-35, the atomic mass is 35 and the atomic number is 17 whereas in Cl-37, the atomic mass is 37 and the atomic number is 17. Due to the existence of isotopes, the atomic mass of chlorine is 35. of electrons but they have different atomic mass. Note: Isotopes are the species in which two or more compounds have the same atomic number or no. The relative atomic mass of chlorine is 35.5. From the above calculation, it is proved that the atomic mass of chlorine is taken as 35.5u. So, now we will calculate the average atomic mass of chlorine i.e. We know that chlorine-35 is found abundantly more than chlorine-37 with a ratio of 3:1 or 75% and 25% respectively. Its atomic mass is relative because chlorine exists in two isotopes having an atomic mass number of 35 and 37 in. For calculating numerical and in many reactions, we use the atomic mass of chlorine is 35.5u because when we calculate the average atomic mass the value comes to be 35.5u. Relative atomic mass of chlorine is 35.5. The atomic mass of the isotopes is 37u and 35u but they have the same atomic number or number of electrons i.e.

Watts to horsepower conversion Infinity Learn. The formation of the isotopes occurs due to the difference in neutrons in the atom because the difference in the neutrons causes a difference in the atomic mass and this is the reason for the formation of the two isotopes of chlorine. Due to the presence of isotopes of chlorine, the atomic mass of chlorine is taken as 35.5u. In nature, there are only two isotopes of Cl which exists i.e. There are two isotopes of the chlorine which have an atomic mass of 35 and 37 and they are found naturally on the earth with a percentage of 75% and 25% respectively. 17 and it is present in the 17 groups of the halogens in period 3.


 0 kommentar(er)
0 kommentar(er)
